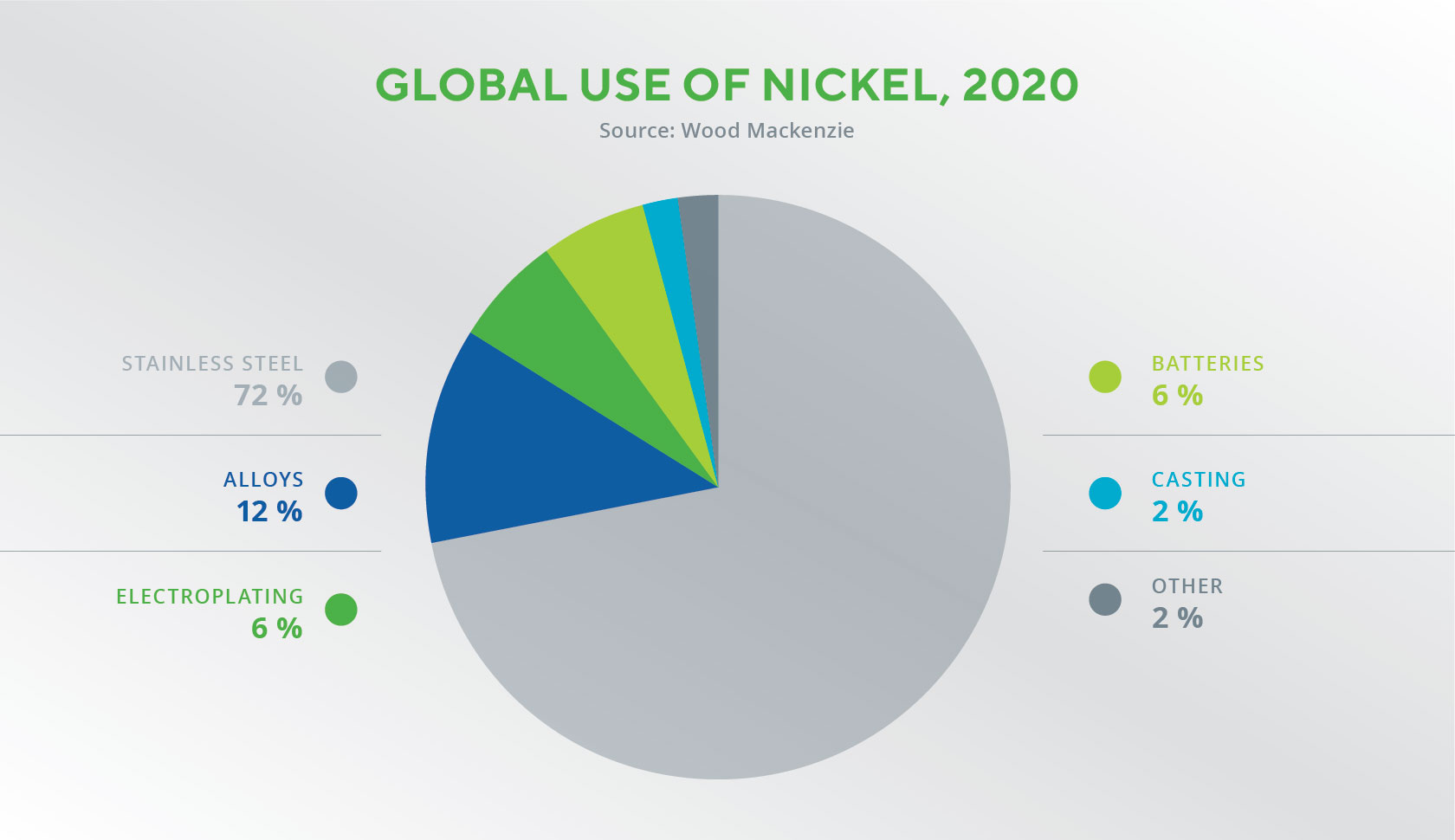
Nickel has many properties that make it versatile in industry and manufacturing and which have turned it into one of our civilisation’s key resources. First of all, it is easy to process – polish, form, forge, weld and roll. Its heat and electrical conductivity are fairly low, and it’s highly resistant to oxidation and corrosion from alkalis, which is why it’s often used to protect the surfaces of other metals, in particular iron. Because nickel has the same oxidation state as iron and many other metals, it can be used as their substitute in various alloys.
Nickel makes up only about 0.008 percent of the Earth’s crust; the vast majority of it can be found in the core. Up to one fifth of the Earth’s core, in fact, is nickel, which plays a key role in maintaining the Earth’s magnetic field and makes nickel the fifth most common element on our planet. Pure nickel can be found also in ores, often together with copper and cobalt. Mineable nickel is most commonly found in magmatic sulphides and laterites; the most important sources of industrial nickel are sulphide deposits created through fractional crystallisation of magma or by ancient lava flows.
Nickel Mining in a Nutshell
Nickel-bearing laterite ores are the result of tropical and subtropical surface erosion, and they account for about 70 percent of dry land nickel deposits. But because extracting nickel from laterites is more expensive than from sulphides, only about 40% of the world’s production comes from laterite sources (Bide et al., 2008). The currently mined sulphide nickel deposits are slowly being depleted, but there are several promising new and highly efficient methods for extraction from laterites on the horizon, such as biological leaching of nickel-bearing ore with chemolithotrophic bacteria. It can be assumed that the share of nickel obtained from laterite deposits will increase (Stanković et al., 2022).
And yet in the early days of industrial nickel mining at the end of the 19th century, before the world started relying on the enormous deposits of sulphide nickel found at the Sudbury Basin in Ontario, Canada, laterites were essentially the only source of nickel in the world. In fact, the actual mining of nickel from laterites tends to be cheaper than from sulphide deposits. While sulphide nickel ores are typically found hundreds of metres underground, laterite deposits can be surface mined, because they rarely extend below 50 metres (Elias, 2002).
On the other hand, the extraction of nickel from laterite ore is more expensive. One reason is that the options for in-situ ore enrichment are very limited and essentially amount to crushing and dehumidifying the ore. The separation of nickel from waste rock is only possible in metallurgic facilities, which means that almost the entire volume of mined ore must be transported there. Compare this to sulphide ores which can be concentrated before smelting by using fairly cheap methods such as selective flotation, or, in the case of ores with high copper content, magnetic separation. This is why the total costs of producing nickel from laterite resources are about 20% higher than in the case of sulphide deposits (Elias, 2002).
The next step in the process is the reduction of nickel oxides through smelting and leaching. Nickel-oxide compounds, however, can also be very useful. For example, nickel oxide hydroxide (NiOOH) is used to make electrodes for nickel-cadmium and nickel-metal hydride cells, and older accumulators also used nickel(II) oxide (NiO). Today this compound is used, for example, as a catalyst in fuel cells, as an ingredient in the manufacturing of many other compounds, and as a traditional glass and porcelain pigment.
An Easily Recyclable Resource
In 2021, the total volume of nickel mined worldwide was about 2.7 million tons. The largest producers are Indonesia with an annual volume of 1,000,000 tons, the Philippines (370,000 t), Russia (250,000 t) and New Caledonia (190,000 t). Other significant deposits can be found in Australia and Canada, with an annual production of 160,000 tonnes and 130,000 tonnes respectively (US Geological Survey, 2022).
Deposits suitable for mining, i.e., those where the average nickel content in the ore is around 1%, still hold at least 300 million tons of nickel, of which about 60% is in laterites and 40% in sulphide sediments (US Geological Survey, 2022). Another large source of nickel is found in manganese nodules, metallic deposits found at the bottom of the ocean which may also contain other metals such as copper, silver, gold, or platinum. The total volume of nickel in these nodules is estimated at another 300 million tons (Bide et al., 2008). But no one is rushing into deep-sea mining, in part because nickel can be fully recycled without losing any of its qualities.
Stainless steel and other nickel alloys are also very durable and tend to be used for a long time. Waste containing nickel can quite easily be identified, separated and reprocessed. In the case of batteries, nickel recycling rates are remarkably high. For example, the efficiency of recycling nickel-cadmium cells in EU countries is between 75% and almost 100% (Eurostat, 2022). While the recovery rate of nickel and steel from industrial nickel-cadmium batteries amounts to over 99.9%. In total, about 65% of currently mined nickel will be recycled, which is undoubtedly impressive (Reck & Graedel, 2012).